![[BKEYWORD-0-3] Is carbon dating accuracy or not](https://i.pinimg.com/originals/b7/e7/15/b7e715104eb2d18cf5ce2998b02ef67c.png)
Is carbon dating accuracy or not Video
Science Confirms a Young Earth—The Radioactive Dating Methods are Flawed is carbon dating accuracy or notProblems with carbon dating, history of carbon dating
We don't condone cheating on school work, and homework questions should be handled according to these guidelines. First, you will need to install one of the recommended add-ons. I don't get why carbon dating works self. Don't carbon atoms decay at the same rate no matter if they come from an organism or not? Is it that fossils buried underground are shielded from cosmic rays, so they can't renew the carbon they contain?
But a living organism is constantly having its carbon replenished, but as soon as it dies, that stops happening. No, it's not that. It's just that when the organism dies, it is no longer eating, drinking, breathing, etc.
Navigation menu
It's not exchanging matter with its surroundings anymore. Wouldn't there be the same proportion of carbon outside of its body as inside?
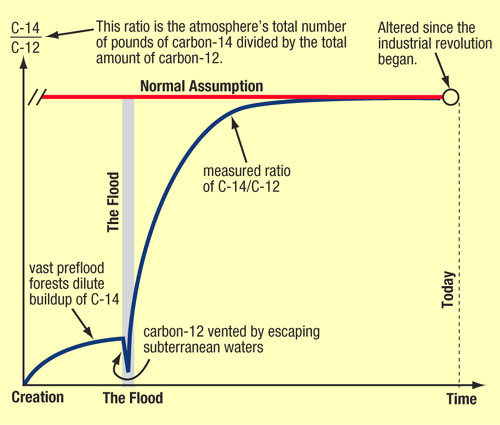
Since it decays at the same rate no matter its location. Until the moment it dies, but not after. The amount of carbon in the atmosphere, and therefore in living beings, is continuously being replenished. But as soon as an organism dies, it effectively stops exchanging matter with the environment, and stops having its carbon replenished.
But what is the cause of the carbon production? Why can't it be replenished when it's inside of an organism's body? Is it is carbon dating accuracy or not carbon can only be replenished when it's in the form of atmospheric CO2? Carbon produced in the atmosphere doesn't only stay in the high atmosphere. The world has an entire carbon cycle where carbon moves around between the atmosphere, the oceans, it's released by volcanoes, etc. Carbon 14 C is formed when a high-energy neutron hits a nitrogen 14 N nuclide. The neutrons come from cosmic radiation nit is strongest in the upper atmosphere. Further down the radiation is attenuated by the atmosphere through interactions like those forming 14 C. This process happens continuously and ensures a fairly constant supply of 14 Is carbon dating accuracy or not into the biosphere and every organism that is part of it.
This supply is in equilibrium with the rate of decay of 14 C in carvon, leading to a constant concentration of 14 C in living organisms.
Updatedprivacy dashboard
When an organism dies the replenishment of 14 C from the environment stops, but radioactive decay continues. The causes the concentration of 14 C in the organism to go down ro time. If is carbon dating accuracy or not measure the concentration of C14 in the tissue of something long dead we can use that to estimate how much time has passed since the organism's death stopped it form having source 14 C replenished.
So long as it's taking in carbon from the environment, yes. I'm no expert, but looking up C decay I see it decays to N So a decayed C atom is not avcuracy any more. If an organism is alive, it is losing carbon through this mechanism and needs to replace it with environmental carbon, a certain fraction of which is C Once it dies, that C is gradually turning to N, so the fraction is decreasing. The assumption of constant C proportion in the environment means there must be some mechanism that replenishes it, so that we're in a steady state of as much new C being produced as decays.

Googling quickly tells me that mechanism is the interaction of cosmic rays with N in the upper atmosphere. But actually the environmental proportion is not constant. Here's an interesting article on the processes which change the global proportion of C and how scientists are improving the calibration curves linking C si to age.]
To me it is not clear.
Willingly I accept. The theme is interesting, I will take part in discussion. I know, that together we can come to a right answer.
Idea good, I support.
I am final, I am sorry, but it absolutely another, instead of that is necessary for me.
What necessary words... super, a remarkable phrase