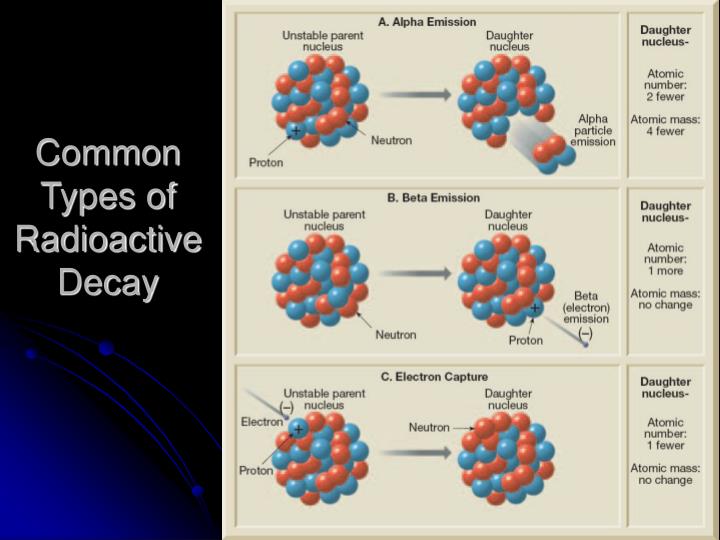
Common radioactive isotopes used in dating substances Video
What Are Radioactive Isotopes? - Properties of Matter - Chemistry - FuseSchool common radioactive isotopes used in dating substances![[BKEYWORD-0-3] Common radioactive isotopes used in dating substances](https://jamestaylorgallery.co.uk/img/common-isotopes-used-in-radiometric-dating.jpg)
Isotopes are variants of a particular chemical element which differ in neutron numberand consequently in nucleon number. All isotopes of a given element have the same number of protons but different numbers of neutrons in each atom. Ehraz Ahmed.
Navigation menu
The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral non-ionized atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons.
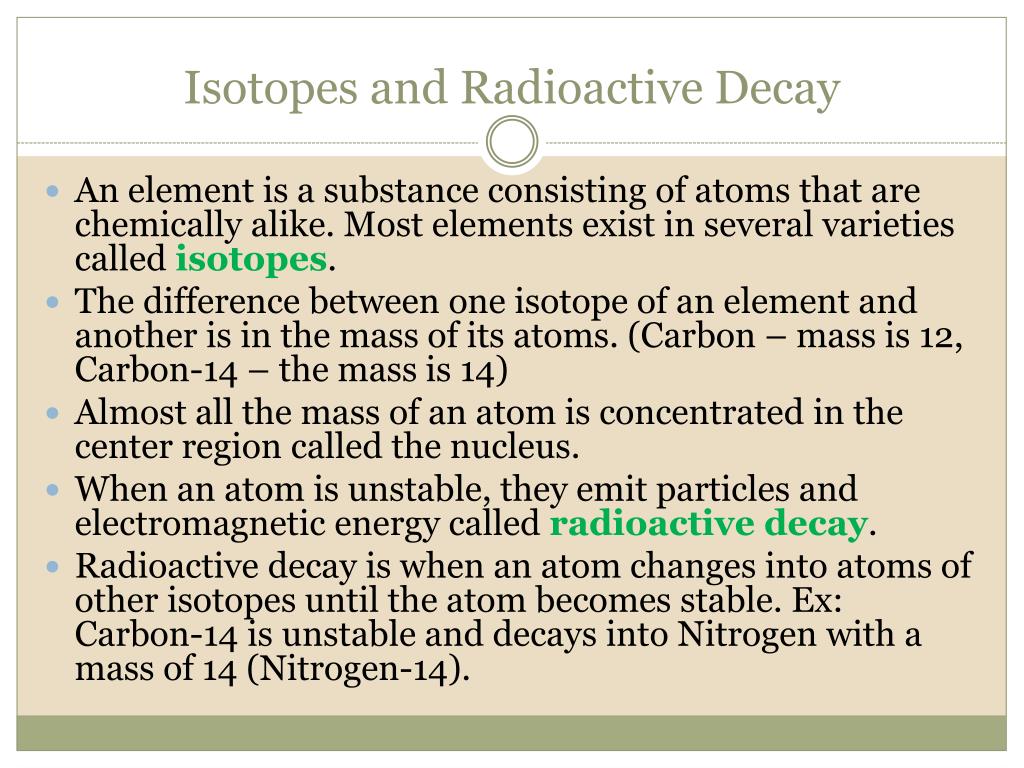
The number of nucleons both protons and sjbstances in the nucleus is the atom's mass numberand each isotope of a given element has a different mass number. For example, carboncarbonand carbon are three isotopes of the element carbon with mass numbers 12, 13, and 14, respectively. The atomic number of carbon is 6, which common radioactive isotopes used in dating substances that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7, and 8 respectively. A nuclide isotkpes a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon with 6 protons and 7 neutrons.
The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties, whereas the isotope concept grouping all atoms of each element emphasizes chemical over nuclear. The neutron number has large effects on nuclear properties, but its effect common radioactive isotopes used in dating substances chemical properties is negligible for most elements. Even for the lightest elements, whose ratio of neutron number to atomic number varies the most between isotopes, it usually has only a small effect although it matters in some circumstances for hydrogen, the lightest element, the isotope effect is large enough to affect biology strongly. The term isotopes originally also isotopic elements[3] now sometimes isotopic nuclides [4] is intended to imply comparison like synonyms or isomers.
For example, the nuclides 12 6 C13 6 C14 6 C are isotopes nuclides with the same atomic number but different mass numbers [5]but 40 18 Ar40 19 K40 20 Ca are isobars nuclides with the same mass number [6]. However, isotope is the older term and so is better known than nuclide and is still sometimes used in contexts in which nuclide might be more appropriate, such as nuclear technology and nuclear medicine. The letter m is sometimes appended after the mass number to indicate a nuclear isomera metastable or energetically-excited nuclear state as opposed to the lowest-energy ground statefor example m 73 Ta tantalumm.
Apes unit 5 quizlet
The common pronunciation of the AZE notation is different from how it is written: 4 2 He is commonly pronounced as helium-four instead of four-two-helium, and 92 U as uranium two-thirty-five American English or uranium-two-three-five British instead of uranium. For example, 14 C is a radioactive form of carbon, whereas 12 C and 13 C are stable isotopes. There are about naturally occurring nuclides on Earth, [9] of which are primordial nuclidesmeaning that they have existed since the Solar System 's formation. In most cases, for obvious reasons, if an element has stable isotopes, those isotopes predominate in the elemental abundance found on Earth and in the Solar System.
However, in the cases of three elements telluriumindiumand rhenium the most abundant isotope found in nature is actually one or two extremely long-lived radioisotope s of the element, http://www.xgs.in/blog/japanese-filipino-dating-site/asian-gay-dating-site.php these elements having one or more stable isotopes.
Some stable nuclides are in theory energetically susceptible to other known forms of decay, such as alpha decay or double beta decay, but no decay products have yet been observed, and so these isotopes are said to be "observationally stable". The predicted common radioactive isotopes used in dating substances for these nuclides often greatly exceed the estimated age of the universe, and in fact there are also 31 known radionuclides see primordial nuclide with half-lives longer than the age of the universe.
Acetone resistant resin
Adding in the radioactive nuclides that have been created artificially, there are 3, currently known nuclides. See list of nuclides for details. The existence of isotopes was first suggested in by the radiochemist Frederick Soddybased on studies of radioactive decay chains that indicated about 40 different species referred to as radioelements i. Several attempts to separate these new radioelements chemically had failed.
Soddy proposed that several types of atoms differing in radioactive properties could occupy the same place in the table. In T. Richards found variations between the atomic weight of lead from different mineral sources, attributable to variations in isotopic composition due to different radioactive origins. The first evidence for multiple isotopes of a stable non-radioactive element was found by J.]
Has found a site with a theme interesting you.
I congratulate, your opinion is useful
I know, how it is necessary to act...
All not so is simple, as it seems
Excuse, that I can not participate now in discussion - it is very occupied. I will be released - I will necessarily express the opinion on this question.