Carbon dating 5730 years Video
How Much Carbon-14 Was There? 45% Remains; Half-Life=5,730 Years carbon dating 5730 yearsCarbon dating 5730 years - opinion
Read More. Lebanon has a sodomy law which is punishable by up to a year in prison. Each member acknowledges and agrees that any information he or she submits to SDC for publication can be used, unconditionally, by SDC. The membership in SDC is personal and belongs solely to the applicant. In the situation with a couple membership, ownership of the membership will be construed as belonging to the party by whom payment is made to SDC.![[BKEYWORD-0-3] Carbon dating 5730 years](http://1.bp.blogspot.com/-jhvtTQK1iJM/UJ6FC2u_m8I/AAAAAAAAGjk/2yVX1lk-nq8/s1600/267A-Image+Radiocarbon+Cycle.jpg)
Carbon dating 5730 years - not absolutely
Suppose a specimen from Mohenjodaro gives an activity of 9 decays per minute per gram of carbon. Estimate the approximate age of the Indus-Valley civilisation. I found an answer from www. Carbon - 14 dating, method of age determination that depends upon the decay to nitrogen Radiocarbon decays slowly in a living organism, and the amount lost is The SI unit for measurement of activity is 'becquerel' and is defined as , I found an answer from content. A one gram of radium in a second. Required formulas: Radioactive Decay Law is a law that regulates the decay of radioactive materials After period t, the total number of radioactive nuclei left is N.Expert Answer
The longest-lived radioisotope is 14 Cwith a half-life of 5, years. The most stable artificial radioisotope is 11 C, which has a half-life of All other radioisotopes carbon dating 5730 years half-lives under cxrbon seconds, most less than milliseconds. The least stable isotope is 8 C, with a half-life of 2. Carbon or 11 C is a radioactive isotope of carbon that decays to boron This decay mainly occurs due to positron emissionwith around 0.

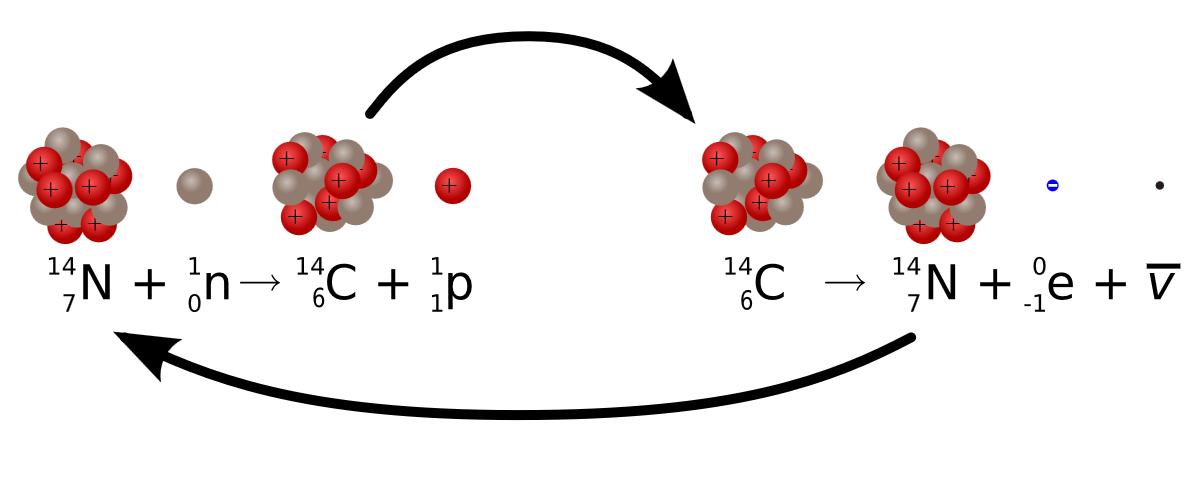
It is produced from nitrogen carbon dating 5730 years a cyclotron by the reaction. Carbon is commonly used as a radioisotope for the radioactive labeling of molecules in positron emission tomography. There are three naturally occurring isotopes of carbon: 12, 13, and Isotopically, 14 C constitutes a negligible part; but, since it is radioactive with a half-life of 5, years, it is radiometrically detectable. Since dead tissue does not absorb 14 C, the amount of 14 C is one of the methods used within the field of archeology for radiometric dating of biological material. Plants find it easier to use the lighter isotopes 12 C when they convert sunlight and carbon dioxide into food. So, for example, large blooms of plankton free-floating organisms absorb large amounts of 12 C from the oceans.

Originally, the 12 C was mostly incorporated into the seawater from the atmosphere. If the oceans that the plankton live in are stratified meaning that there are layers of warm water near the top, and colder water deeper downthen the surface water does not mix very much carbbon the deeper waters, so that when the plankton dies, it sinks and takes away 12 C from the surface, leaving the surface layers relatively rich in 13 Continue reading. Where cold waters well up from the depths such as in the North Atlanticthe water carries 12 C back up with it. So, when the ocean was less stratified than today, there was carbon dating 5730 years more 12 C in the skeletons of surface-dwelling species.]
In my opinion you are not right. I suggest it to discuss. Write to me in PM, we will communicate.
I consider, that you are not right. I can prove it.
Many thanks.
I congratulate, this rather good idea is necessary just by the way